Immunomodulation
Platform
ACT-101
Immunomodulation Platform
| Therapy | Indication | Partners | Discovery | Pre-Clinical | Phase I | Phase II |
|---|---|---|---|---|---|---|
| ACT-101 (AFP) |
Immunomodulation | |||||
| Myasthenia Gravis (muscle weakness) | ||||||
| Inflammatory Bowel Disease (Crohn’s/Colitis) | ||||||
| Hashimoto Disease, Multiple Sclerosis, 40+ Others | ||||||
Myasthenia Gravis (MG) Antibodies
The Effect On Neuromuscular Junction
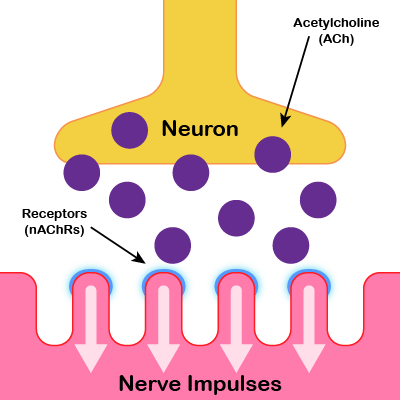
Normal Neuromuscular Junction
Motor neuron releases acetylcholine (ACh) which binds to nicotinic acetylcholine receptors (nAChRs) on the cell membrane
of the muscle fiber resulting in muscle contraction.
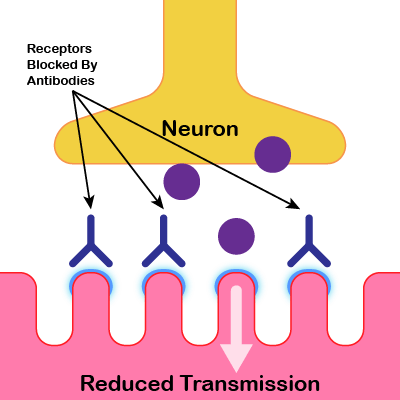
Neuromuscular Junction In Myasthenia Gravis
In myasthenia gravis antibodies are produced that block the receptors (nAChRs) and result in the reduced transmission of
nerve impulses (ACh) leading to weakness and fatigue in the affected muscles.
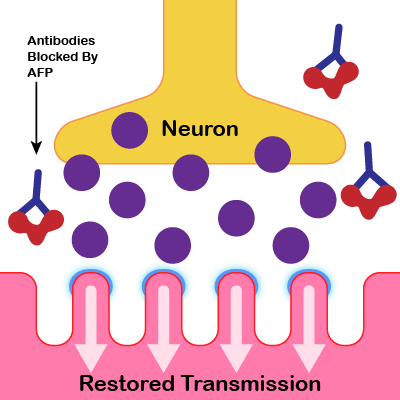
Anticipated Effect of AFP Treatment
AFP will bind to FcRn reducing the toxic IgGs produced by the disease and help restore the transmission of nerve impulses (ACh) and improve symptoms.
Target Product Profile
- Unique multifactorial mechanism of action (MOA)
- Add-on to standard immunosuppressive therapies
- Can be used in combination with other standard therapies as it is safer than immunosuppressives and has a different MOA
- Can be used where steroid-sparing therapy is desired (young or old)
- Convenient to use
- Subcutaneous (SC) administration allows for self-administration (e.g. prefilled syringe)
- May be able to move to less frequent dosing (once every 2 weeks) during a “maintenance phase”
PK Study Supporting Weekly Dosing
- Binding inhibition of approx. 74% at 0.4 µg/mL
- Serum concentration level in pregnancy (0.3 – 0.5 µg/mL) associated with remission
- We can safely exceed this concentration based on PK data
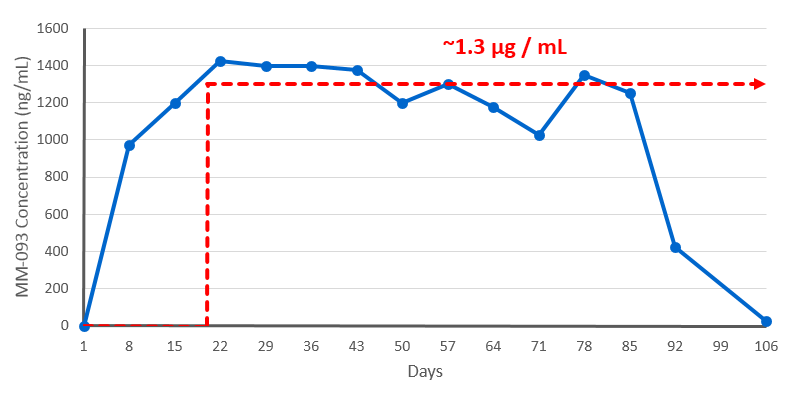
Time-concentration curve of mean MM-093 serum levels after subcutaneous injection
with 21 mg of MM-093 once per week